"Tiny Patients, Big Strides: The Rise of Robotic Surgery in Pediatric Care"
- Dr Vivek Viswanathan

- Mar 26, 2024
- 7 min read
Updated: Apr 11, 2024
Imagine a world where surgeons use miniature robotic assistants to perform complex procedures on children, minimizing scarring and recovery times. This isn't science fiction; it's the exciting reality of robotic surgery in pediatric care.
The word "robot" first entered our vocabulary in 1921, appearing in a Czech play titled "Rossum's Universal Robots." While the concept of robots captured imaginations early on, their application in medicine took time. It wasn't until 1985 that the first medical procedure utilizing a robot was performed, assisting surgeons in brain biopsies.
Fast forward to the year 2000, and the landscape of surgery shifted significantly. The US Food and Drug Administration (FDA) approved the Da Vinci Surgical System, developed by Intuitive Surgical. This marked a turning point, paving the way for wider adoption of robotic technology in operating rooms. Just one year later, history was made again with the first robotic prostatectomy being performed.

Breaking Barriers:
The 2010s saw a surge in robotic surgery for pediatric urology. Procedures like pyeloplasty (correcting a blockage in the ureter) and pyelonephrotomy (removing kidney stones) became prime candidates for robotic assistance. This decade also witnessed the development of smaller, more adaptable robotic instruments specifically designed for pediatric applications.
Today, robotic-assisted surgery has become a well-established tool in the field of pediatric urology and pediatric surgery as a whole. Surgeons are leveraging this technology to offer minimally invasive approaches to a growing range of procedures, improving outcomes for young patients.


Our exploration of robotic surgery in pediatrics brings us to the technology itself. A web search reveals a diverse landscape, with at least fifteen distinct robotic platforms identified. Seven of these platforms have received authorization for clinical use within various healthcare systems.
These authorized platforms include:
Medtronic Hugo™ RAS
Cambridge Medical Robotics Versius®
Intuitive Surgical Da Vinci SP®
Medrobotics Corp. Flex Robotic System
Asensus Senhance® ALF-X
Meerecompany Inc. Revo-i™
Wego Micro Hand S
It's important to note that clinical results for these platforms have been reported, highlighting their growing use and potential benefits in pediatric surgery.


While seven robotic platforms have secured authorization for clinical use in pediatrics, several others are on the horizon. Five such systems are currently authorized, but their use in pediatric surgery hasn't been documented in scientific literature yet.
These include:
Medicaroid Hinotori™
Avatera Medical Avatera®
Distalmotion Dexter
Moon Surgical Maestro
Virtual Incision MIRA
Our exploration also revealed three additional surgical platforms in development. However, details regarding their clinical approval status or specific applications in pediatric surgery are currently unavailable. These platforms are:
Titan Medical Inc ENOS™
SS Innovation Mantra
Rob Surgical Systems S Bitrack System
Available information on the existing robotic platforms:
Clinically Adopted Platforms | ||||
Company | Product Name | Country | Regulatory Approvals | Marketing Information (n. Procedures/Platform) |
Medtronic | Hugo™ RAS | US | FDA: ongoing CE-mark: general surgery; urology; gynecology Australian TGA: urology; gynecology Health Canada: general surgery MHLW PMDA Japan: urology; gynecology | NR |
Cambridge Medical Robotics | Versius® | England | CE-mark: general surgery; urology; gynecology; thoracic surgery Australian TGA: general surgery; urology; gynecology Anvisa Brazil: general surgery; urology; gynecology Other countries: India; Pakistan; Egypt | |
Intuitive Surgical | Da Vinci SP® | US | FDA: urology; transoral procedures MHLW PMDA Japan: urology; gynecology; general surgery; thoracic surgery; transoral MFDS Korea: urology; general surgery; gynecology; thoracic surgery; transoral NMPA China: yes, not specified | |
Medrobotics Corp. | Flex® Robotic System | US | FDA: transoral; colorectal; general surgery; gynecology; thoracic surgery CE-mark: colorectal Australian TGA: colorectal | Bankrupt of the producing company |
Asensus (formerly TransEnterix) | Senhance® ALF-X | US | FDA: general surgery; gynecology. Pediatric surgery expected in 2023 CE-mark: general surgery; gynecology; pediatric surgery MHLW PMDA Japan: urology; gynecology; general surgery; thoracic surgery Roszdravnadzor—Russia: yes, not specified Taiwan: yes, not specified | |
Meerecompany Inc. | Revo-i™ | South Korea | MFDS Korea: urology; gynecology; general surgery | NR |
Wego | Micro Hand S | China | NMPA China: general surgery | Reddot award winner 2022 |
Platforms under Clinical Investigation | ||||
Company | Product Name | Country | Regulatory Approvals | Marketing Information |
Medicaroid | Hinotori™ | Japan | MHLW PMDA Japan: urology; gastrointestinal; gynecology | |
Avatera Medical | Avatera | Germany | CE-mark: urology; gynecology | Fist clinical procedure in May 2022 [119] |
Distalmotion | Dexter | Switzerland | CE-mark: general surgery; gynecology | |
Moon Surgical | Maestro | US | FDA: laparoscopic procedures CE-mark: laparoscopic procedures | 30 procedures performed [121] |
Virtual Incision | MIRA | US | FDA: completed IDE for bowel resections. De novo classification pathway ongoing | NR |
Titan Medical Inc. | ENOS™ (formerly SPORT) | Canada | FDA: planned in 2023 CE-mark: planned in 2023/24 | NR |
SS Innovation | Mantra | India | FDA: planned in 2023 CE-mark: planned in 2023 Other countries: India | |
Rob Surgical Systems S | Bitrack System | Spain | NR | First clinical trial ongoing [123] |
US: United States; FDA: food and drug administration; CE: Conformité Europeenne; TGA: Therapeutic Goods Administration; MHLW PMDA: Ministry of Health, Labour and Welfare Pharmaceuticals and Medical Devices Agency; NR: not reported; MFDS: Ministry of Food and Drug Safety; NMPA: National Medical Products Administration; IDE: Investigational Device Exemption.

A Diverse Landscape: Robotic Platform Designs
The seven authorized robotic platforms for pediatric surgery showcase a variety of designs and functionalities:
Modular Design with Independent Arms (5 Systems): Platforms like Medtronic Hugo RAS and Asensus Senhance ALF-X feature a modular design with independent arms, typically ranging from three to four, including the dedicated optical arm for visualization. This allows for greater flexibility and maneuverability within the operating field.
Multi-arm Architecture (5 Platforms): Several platforms, including Meerecompany Revo-i and Medicaroid Hinotori, employ a multi-arm architecture. Similar to the modular design, these systems have three to four arms, including the optical arm.
Single-Port Platforms with Flexible Arms (2 Systems): Intuitive Surgical's Da Vinci SP represents a unique approach with its single-port design. This platform utilizes three to four flexible arms, including the optical arm, for operating through a single incision.
Miniaturized Design for Single-Access Surgery (1 Platform): The Virtual Incision MIRA platform stands out with its miniaturized design. This innovative system allows for the insertion of two sterile arms and the optical components directly through a single incision, minimizing invasiveness.
Flexible Endoscope with Operating Arms (1 Platform): The Medrobotics Flex Robotic System offers a distinct approach. It functions as a flexible endoscope equipped with two integrated operating arms.
Laparoscopic Instrument Assistance System (1 System): Moon Surgical's Maestro platform deviates from the typical robotic surgical concept. It's designed to assist surgeons during laparoscopic procedures by providing support and positioning for laparoscopes and other laparoscopic instruments.
This diversity in robotic platform designs highlights the ongoing advancements in minimally invasive surgery for pediatric patients. Each platform offers distinct advantages depending on the specific procedure and the surgeon's needs.
Summary of the overall characteristics of the robotic platforms.
Robotic Platform | Patient Cart Architecture | Console Architecture | Operative Arms No. | Trocars | Instruments | Instruments’ Reusability | Advanced Energy |
Medtronic Hugo™ RAS | Modular | Open | 3 | Commercial | Wristed | Reusables (some disposables) | NA |
Cambridge Medical Robotics Versius® | Modular | Open | 3 | Commercial | Wristed | Reusables | NA |
Intuitive Surgical Da Vinci SP® | Single port | Closed | 3 | Dedicated + commercial | Wristed | Reusables | NA |
Medrobotics Corp. Flex® Robotic System | Flexible system | / | 2 | / | Wristed | Disposables | NA |
Asensus Senhance® ALF-X | Modular | Open | 3 | Commercial | Rigid with a kit of wristed | Reusables | Ultrasonic (rigid) |
Meerecompany Inc. Revo-i™ | Multiarm | Closed | 3 | Commercial | Wristed | Reusables | Ultrasonic (rigid) |
Wego Micro Hand S | Multiarm | Open | 2 | Dedicated | Wristed | Reusables | Ultrasonic (rigid) |
Medicaroid Hinotori™ | Multiarm | Semi-open | 3 | Dedicated | Wristed | Reusables | NA |
Avatera Medical Avatera | Multiarm | Semi-open | 3 | NR | Wristed | Disposables | NA |
Distalmotion Dexter | Modular | Open (with laparoscopic screen) | 2 | Commercial | Wristed | Disposables | NA |
Moon Surgical Maestro | Multiport instrument holder | / | 1 | Commercial | / | / | NA |
Virtual Incision MIRA | Single port | Open | 2 | NR | Wristed | Reusables | NA |
Titan Medical Inc. ENOS™ (formerly SPORT) | Single port | Open | 2 | NR | Wristed | Reusables | NA |
SS Innovation Mantra | Modular | Open | 3 | Dedicated | Wristed | Reusables | NA |
Rob Surgical Systems S Bitrack System | Multiarm | Open | 3 | Commercial | Wristed | Disposables | NA |
NR: not reported; NA: not available.
Surgeon Console:
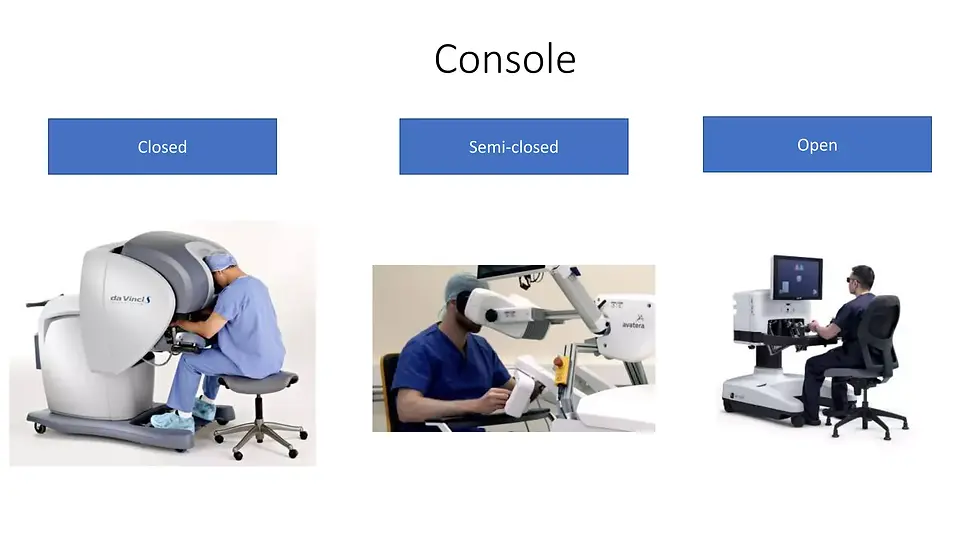
Surgeon Interface: Viewing the Operating Field
The way surgeons interact with these robotic platforms varies across the different designs:
Open Console (Majority - 64.3%): Most platforms, including Medtronic Hugo RAS and Asensus Senhance ALF-X, utilize an open console design. This setup allows surgeons a clear view of the operating field through a high-definition monitor positioned at a comfortable distance. One exception within this category is the Distalmotion Dexter system. While it has an open console, it doesn't have a dedicated viewing system. Instead, surgeons rely on a standard laparoscopic screen positioned within the sterile field.
Closed Console (Da Vinci-like Architecture - 2 Systems): Platforms like Intuitive Surgical's Da Vinci SP employ a closed console design similar to traditional Da Vinci systems. Surgeons view the operating field through a magnified, high-resolution display within the enclosed console.
Semi-open Console (2 Systems): The Avatera Medical Avatera and Medicaroid Hinotori platforms offer a compromise between open and closed consoles. This semi-open design provides surgeons with an immersive view through a dedicated viewer but avoids the bulkiness of a fully enclosed console, potentially reducing the feeling of isolation during surgery.
Direct Control Interface (1 System): The Medrobotics Flex Robotic System takes a unique approach. It forgoes a traditional console altogether. Instead, surgeons directly control the endoscope and two mechanical arms using an open, two-dimensional screen. This eliminates the complexity of electromechanical mediation.
Instrument Holder System (1 System): Moon Surgical's Maestro platform deviates from the concept of a surgeon console entirely. It functions primarily as a holder for laparoscopes and other laparoscopic instruments, offering surgeons improved control and positioning during laparoscopic procedures.
This variety in console designs caters to surgeon preferences and the specific requirements of minimally invasive pediatric surgery.
Accessing the Operating Field: Trocars and Instruments
The methods for accessing the surgical field differ across these robotic platforms:
Standard Laparoscopic Trocars (Majority: 7): Most platforms, including Medtronic Hugo RAS, Asensus Senhance ALF-X, and Cambridge Medical Robotics Versius, utilize familiar laparoscopic trocars to create access points for instruments.
Dedicated Trocar Systems (Several Platforms: 4): Several platforms, like Wego Micro Hand S and Medicaroid Hinotori, employ dedicated trocars designed specifically for their system, potentially offering improved ergonomics or functionality.
Single-Site Access with Da Vinci SP: The Intuitive Surgical Da Vinci SP utilizes a dedicated metallic trocar compatible with a disposable commercial single-site access system, minimizing the number of incisions needed.
Trocar-Free Access with Medrobotics Flex: The Medrobotics Flex Robotic System operates similarly to a colonoscope, eliminating the need for trocars altogether, offering a potentially less invasive approach.
Instrument Choices for Delicate Procedures
The type of instruments used also varies across platforms, catering to the needs of minimally invasive pediatric surgery:
Wristed or Flexible Instruments (Most Systems): Most platforms, like Intuitive Surgical Da Vinci SP and Meerecompany Revo-i, offer wristed or flexible instruments for enhanced dexterity and maneuverability within the confined operating space.
Limited Wrist Articulation with Asensus Senhance (Optional): The Asensus Senhance ALF-X platform may be compatible with wristed instruments from specific brands like Radia, although this might not be universally available for all surgeons.
Specialty Instruments for Wego Micro Hand S: Some configurations of the Wego Micro Hand S system include a rigid, advanced ultrasonic dissector, potentially offering additional functionalities for specific procedures.
Reusability: Balancing Cost and Efficiency
The reusability of instruments differs depending on the platform, impacting cost and efficiency considerations:
Reusable Instruments (Dominant Approach): A significant number of platforms, including Intuitive Surgical Da Vinci SP and Meerecompany Revo-i, utilize reusable instruments after proper sterilization, offering a potentially cost-effective approach in the long run. One system, Virtual Incision MIRA, stands out for being entirely sterilizable and portable.
Partially Reusable with Medrobotics Flex: The Medrobotics Flex Robotic System employs a combination of reusable and disposable instruments, potentially offering a balance between cost and convenience.
Disposable Instruments (Some Systems): Platforms like Avatera Medical Avatera and Distalmotion Dexter rely solely on disposable instruments, simplifying instrument handling but potentially increasing overall surgical costs.
Mixed System with Medtronic Hugo RAS: Medtronic Hugo RAS uses a combination of sterilizable instruments and disposable tools for specific functions like needle driving and cutting, offering flexibility based on the surgical needs.
Laparoscopic Instrument Compatibility with Moon Surgical Maestro: Moon Surgical's Maestro platform doesn't have dedicated robotic instruments; instead, it allows surgeons to use standard laparoscopic instruments, leveraging their existing inventory.
Advanced Energy Options: Beyond Basic Functions
While all platforms support basic monopolar and bipolar energy for tissue manipulation, advanced features like ultrasonic energy are limited:
Advanced Ultrasonic Energy (Limited Availability): Only a select few platforms, including Asensus Senhance ALF-X and Meerecompany Revo-i, currently offer advanced ultrasonic energy capabilities, potentially offering benefits like improved tissue dissection and reduced blood loss.
Staplers and Advanced Energy Availability: A Work in Progress
It's important to note that a complete range of staplers or advanced energy options isn't yet available for all the platforms reviewed. As technology continues to evolve, we can expect these functionalities to become more widely adopted across the spectrum of robotic surgery platforms for pediatric applications.
The Rise of Robotic Surgery Across Surgical Specialties:
The use of robotic technology is rapidly expanding in the field of paediatrics beyond its initial applications in urology and general paediatric surgery. Recent publications describe successful applications of novel robotic devices in a wide range of procedures, including hepatobiliary surgery, colorectal surgery, abdominal wall reconstruction, upper gastrointestinal surgery, endocrine surgery, and even breast surgery.
Early promise, unanswered questions:
While these initial reports are encouraging, the overall evidence base remains modest. Most studies suggest the feasibility of performing these procedures robotically, with minimal technical limitations reported. Additionally, with several new platforms receiving regulatory approval, the Asian market is likely to fuel further development in this field.
Standardization and training hurdles:
However, there are significant challenges to address before widespread adoption. Currently, there's no standardized international training curriculum or credentialing program for robotic paediatric surgery. This lack of standardization makes it difficult to assess surgical proficiency and hinders the transferability of skills between different robotic systems.
Looking ahead:
Despite these challenges, the future of robotic paediatric surgery appears bright. Technological innovation is poised to continue at a rapid pace, offering exciting possibilities across a spectrum of surgical specialties. However, robust clinical studies are needed to fully evaluate the long-term benefits and cost-effectiveness of these new applications.
Credits:
Marchegiani F, Siragusa L, Zadoroznyj A, Laterza V, Mangana O, Schena CA, Ammendola M, Memeo R, Bianchi PP, Spinoglio G, Gavriilidis P, de'Angelis N. New Robotic Platforms in General Surgery: What's the Current Clinical Scenario? Medicina (Kaunas). 2023 Jul 7;59(7):1264. doi: 10.3390/medicina59071264. PMID: 37512075; PMCID: PMC10386395.















Comments